Note: Pump systems for life-supporting medication, such as cardiac medication, are not the subject of this overview.
Alarm-free and safe
Electronic infusion pumps have alarm systems that indicate malfunctions. They are equipped with sensors that should immediately detect a malfunction or hazards, e.g. air inclusions or an interruption in the flow of the medication. These sensors are deliberately set to be very sensitive, so that false alarms occur quite often. This bears the risk that these will no longer be taken account of.4
Protection against air embolisms
Elastomeric pumps do not require an alarm according to ISO 28620. The specifications for safety features that must be included in every pump are strict. With electronic pumps, an air sensor stops the infusion and an alarm is triggered. With elastomeric pumps, the elastomeric reservoir has no dead space and even if some air enters during filling, it is completely removed by inline filters with forced venting. These eliminate any air inclusions. Particularly safe pumps have a second air filter after additional modules such as a PCA bolus module. The second air filter eliminates any possible small air inclusions from such modules. Generally, air filters have a maximum pore size of 0.2 μ and are thus bacteria-tight.
A so-called venting cap on the patient connection offers additional protection: when filling, the line automatically vents via a bacteria-tight air filter in the cap completely. The system remains sterile. Contamination of the system by opening and closing the end cap or contamination of the workplace by leaking drops can thus be avoided. The last point is particularly important for applications with cytostatics.
Particle free infusion
According to ISO 28620 a particulate filter, as a further safety feature, must be integrated in every elastomeric pump. It filters particles that can get into the solution during preparation, for example. The finer the particulate filter, the more or smaller particles are filtered out of the solution, the more effective the protection.
Reliable drug delivery
Inside the pump housing, controlled expansion and contraction of the balloon is optimal. With superior elastomeric pumps, this is achieved by means of a guided system integrated in the housing. This keeps the balloon centred when filling with the medication and is thus expanded evenly.
In addition it improves the accuracy of the progress indicator, which enables a more precise reading of the pumping progress. With so-called soft-shell pumps, where the casing around the balloon is soft, the progress of pumping is not well recognisable. Occlusions of the patient access, temporary or permanent, are only detected with elastomeric pumps by monitoring the progress. With pumps that have a detailed progress indicator, permanent occlusions can be detected early.
A stop of the medication flow due to kinking of infusion lines also triggers an alarm with electronic pumps. With premium elastomeric pumps, this is unnecessary due to kink-resistant lines. Particularly safe infusion lines are made of thicker material and have irregular clearing, e.g. triangular, which allow a medication flow even in the case of a complete kink.
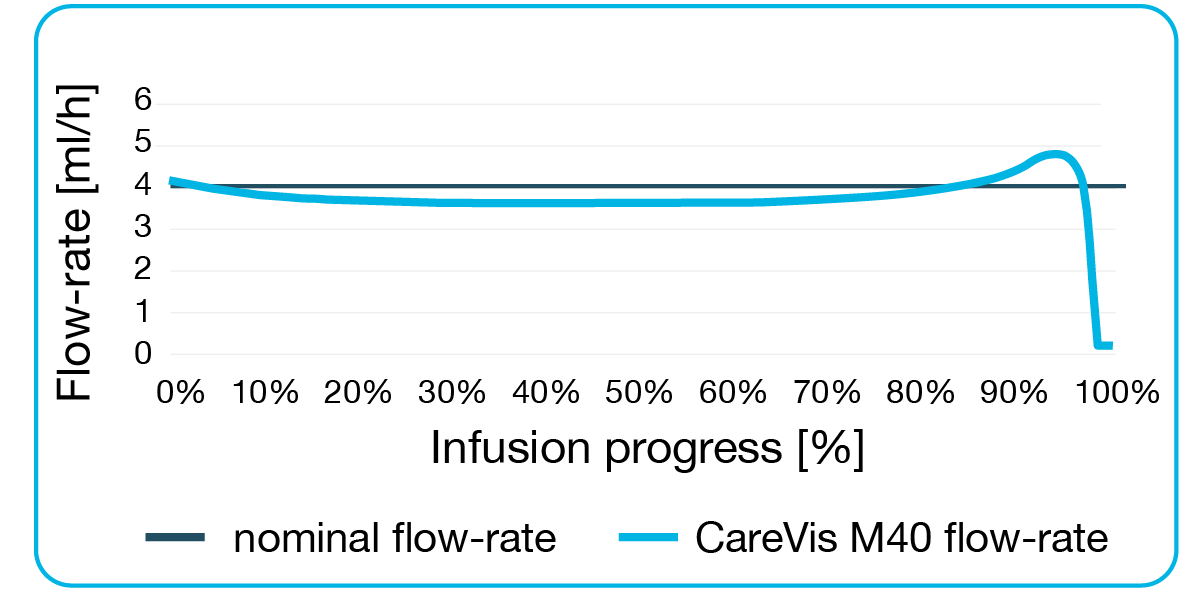
When emptying the mechanical pump, the flow-rate is controlled by physical laws. The requirements for determining the flow-rate as well as permissible tolerances are regulated by ISO standard 28620. Elastomeric pumps are available with different basal rates for very slow or faster drug delivery, according to the needs of the therapy. Depending on the quality of the elastomeric pumps, an average flow-rate of ±15 to ±10% to the nominal flow-rate is achieved (measured according to ISO 28620). Since the standard only considers the average flow-rate, higher deviations are possible while the infusion is in progress. With high-quality elastomeric pumps the applied infusion pressure of the balloon is relatively constant. They meet the flow-rate at all times and do not just average the nominal curve. Therefore, the deviation of short-term measured flow-rates from the nominal flow-rate is relatively small.Digital security gaps
No incorrect programming
With electronic infusion pumps, different protocols for different applications can be programmed. This creates additional risks. One of the most common causes of medication errors is misprogramming.3 Since elastomeric pumps are not programmed, no incorrect programming is possible.7 Volume, flow-rate and, if applicable, bolus data are defined by the selected pump. In a single-use system, the pump that is best suited to the application can always be used. For those cases that require readjustment of the flow-rate, elastomeric pumps with adjustable flow-rate are offered. High-quality products have a clearly readable display.
Safe from external interference
In the past, several security gaps have been discovered in electric pump systems.1 The pumps were accessible from the outside and the settings were able to be changed. In an emergency, the lives of patients could be endangered. Some security gaps have been eliminated through software updates. As is generally required for digital systems, the security of digital infusion pumps also requires constant updates of the software. Elastomeric pumps are safe from hacker attacks. Since they function purely mechanically according to physical laws, they cannot be manipulated.
Free of power and batteries
An electronic pump requires an external power source to operate. Elastomeric pumps function independently, without electricity or battery. This ensures that the infusion can run reliably to the end.5 There is no pump stop due to empty batteries or lack of electricity. There is therefore also no risk of fire from leaking fluid hitting electricity.2
Safety for particular therapies
Patient Controlled Analgesia (PCA)
Common models of bolus units consist of the bolus reservoir, which is squeezed forward by pressing the bolus button, of a restrictor capillary before the reservoir, which determines the filling time of this reservoir by the predetermined flow rate (corresponds to the lock-out time of electronic pumps), and of the valve behind the reservoir, which prevents free flow. Due to the selected volume of the flexible reservoir below the bolus button, the maximum retrievable bolus dose is predefined. The time-delayed filling of the reservoir after emptying, effected by fine restrictor capillary at the reservoir inlet prevents overdosing of medication by the patient beyond the maximum intended dose. Another safety feature of elastomeric pumps corresponding to the lock-out time as of electronic pumps and which moreover cannot be tampered in any way. The safety of elastomeric pumps for patient-controlled analgesia (PCA) (PCA) depends on the quality and mechanism of those modules. In many models, pressing on the bolus button actuates a lever mechanism which opens the valve behind the reservoir. A safety risk: The lever can stick and the exit of the reservoir is not closed again. As a result the maximum possible bolus volume, determined by the restrictor capillary in front of the bolus reservoir, is continuously administered, even if this is not requested by the patient. Pumps with an elastic bolus button and elastic pressure relief valve, which is only opened by brief pressure and automatically falls back into the closing position after the reservoir is emptied, are safer, even if the button is held pressed.
Safe administration of cytostatics
A certain risk in the administration of cytostatics is delays in the flow rate due to 5-FU precipitation in the flow restrictor. Elastomeric pumps with a modified flow restrictor in wide-channel technology ensure delay-free flow and prevent blockages caused by fine crystalline 5-FU precipitates. With wide-channel technology, the flow channel is larger in cross-section than that of conventional flow restrictors with an equivalent flow rate. Another risk, the influence of medication by UV radiation, is prevented by UV-blocking outer material of high-quality pumps.
Neuraxial applications
Elastomeric pumps are also available with a so-called neuraxial connector according to ISO 80369-6. Neuraxial connectors are usually distinguished by their yellow coloured patient connector and are not compatible with Luer-connectors. A double safety feature that does not allow any confusion when connecting the pumps.
Using elastomeric pumps safely
Elastomeric infusion pumps are easy to use. For safe use, however, you should familiarise yourself with the operating principles of an elastomeric pump and observe the calibration requirements of the respective manufacturer. Elastomeric pumps are an active medical device and may be subject to instruction by the seller according to national guidelines.
Avoid impairment of the flow rate
Elastomeric pumps are not gravity infusions. The pump should be placed at the same height as the patient connection. Positioning it too high or too low would result in a faster or slower flow rate. As the pumps work by mechanically deflating the elastomeric balloon, it must be specially protected. External pressure would cause a faster flow rate. This can happen, for example, with soft-shell pumps, where the elastomeric balloon is only covered by a soft-shell if the patient accidentally lies down on the pump while asleep.6 A hard-shell (so-called hard-shell pumps) protects the balloon from external pressure.
Observe temperature
For safe use, the temperature of the medication must also be observed. Temperature fluctuations can influence the delivery of the medication, as even small changes in temperature can change the viscosity of the solution. Therefore, the ambient temperature should be as constant as possible during infusion. Instructions should be found in the manufacturer‘s instructions for use.
Safety features of CareVis
CareVis is a maintenance-free medical product manufactured according to the current state of the art and with high-quality materials. The pumps have numerous integrated safety features. The wide range with volumes from 60 ml to 600 ml, multiple flow-rates from 1 ml/h to 250 ml/h, plus modules for patient-controlled analgesia - with or without basal-rate - and models with adjustment of the basal flow-rate, meet a variety of requirements for protocols in everyday clinical use. Pumps with additional modules are each equipped with an air and particle filter before and after the module - a unique advantage for patient safety. Stability data for frequently used medicines and solutions are available, which we are happy to provide.
The safety features of CareVis at a glance:
- precise flow-rates - only +/- 10% deviation, progression relatively constant
- particle filter of 1.2 μ and ventilation filter of 0.2 μ
- bacteria-proof venting cap - priming with the cap closed without dripping
- closed one-way system
- kink-resistant infusion line
- reinforced, break-proof casing
- transparent casing - flawless drug inspection
- UV blocking capabilities
- easy to read progression indicator
- guided balloon
- independent of external energy sources
- latex and phthalate free
- all CareVis models are available with a Luer-lock or with a neuraxial connector
The safety features of CareVis PCA pumps:
- Bolus units, predefined and programmable with: elastic overpressure valve that closes immediately after emptying the reservoir. Even when the bolus button is pressed and held down
- accurate bolus volumes and precise filling times (lock-out time)
- elastic bolus button, does not get stuck
The safety features of CareVis OncO pumps:
- Modified flow limiter in wide-channel technology
We offer personal instructions on our pumps as well as a wide range of information material for medical doctors, healthcare professionals or patients. Please do not hesitate to contact us!
Download
Sources
1 McAfee Enterprise: Kritische Schwachstellen in medizinischen Infusionspumpen entdeckt (zuletzt 28.03.2022)
2 Bundesinstitut für Arzneimittel und Medizinprodukte (BfArM): Sicherer Betrieb von Infusionspumpen (zuletzt 28.03.2022)
3 Stiftung für Patientensicherheit: Dosierungsfehler trotz Infusionspumpen und Infusionsspritzenpumpen, Quelle: 2. Institut of Healthcare Improvement. Reduce adverse drug events (ADEs) involving intravenous medications: implement smart infusion pumps. (zuletzt 28.03.2022)
4 WEKA Fachmedien GmbH: Ungestörte Infusion (zuletzt 28.03.2022)
5 Benrath, A.-K. B., Blunk, J., Söhle, S., Feldmann, R., & Bauer, M. (10 2014): Kostenminimierungsanalyse in der postoperativen Schmerztherapie. Der Anaesthesist, S. 783-792.
6 Thiveaud, D., Demazières, V., & Lafont, J. (02 2005). COMPARISON OF THE PERFORMANCE OF FOUR ELASTOMERIC DEVICES. IndustryScience, S. 54-56.
7 S.L., R., Rowbotham, D., & Mushambi, M. (1992). Electronic and disposable patient- controlled analgesia systems. A comparison of the Graseby and Baxter systems after major gynaecological surgery. Anaesthesia, 47:161-3.
